Crystal engineering of a series of complexes and coordination polymers based on pyrazole-carboxylic acid ligands†
Abstract
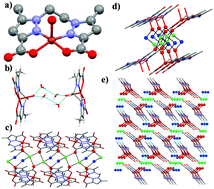
Three new complexes namely [Cu(L1)H2O]·2H2O (1), [Cu2(L4)2(DMF)2(H2O)2](NO3)2 (4) and [{Cd2(L4)2Cl2(DMF)2}] (5), and two coordination polymers namely [Co(μ-L2)(DMF)2]·2H2O (2) and [Co(μ-L3)(DMF)2]·H2O (3) have been synthesized from a series of pyrazole-carboxylate ligands namely 1,1′-(ethane-1,2-diyl)bis(5-methyl-1H-pyrazole-3-carboxylic acid) (H2L1), 1,1′-(oxybis(ethane-2,1-diyl))bis(5-methyl-1H-pyrazole-3-carboxylic acid) (H2L2), 1,1′-(butane-1,4-diyl)bis(5-methyl-1H-pyrazole-3-carboxylic acid) (H2L3), and 2-(1′,5,5′-trimethyl-1H,1′H-[3,3′-bipyrazol]-1-yl)acetic acid (H2L4), and CuII/CdII/CoII ions. Single crystal structures of these compounds are discussed in the context of the effect of conformation of the ligands and supramolecular interactions observed within these edifices. Temperature-dependent magnetic data of 4 could be fitted best using two non-interacting CuII ions (S = 1/2) per molecule with giso = 2.28 and a temperature-independent paramagnetism, TIP = 1.75 × 10−5 cm3 mol−1.


 Please wait while we load your content...
Please wait while we load your content...