Se reduction by CO/H2O in ionic liquid: acceleration effect by activating water molecules†
Abstract
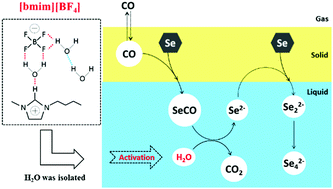
To improve the safety and efficiency of the Se/CO/H2O redox process, five ionic liquids (1-butyl-3-methylimidazolium tetrafluoroborate, [bmin][BF4], 1-propyl-3-methylimidazolium tetrafluoroborate, [pmim][BF4], 1-ethyl-2,3-dimethylimidazolium tetrafluoroborate, [bdmim][BF4], N-ethylimidazolium tetrafluoroborate, [eim][BF4], and 1-butyl-3-methyl-imidazolium trifluoromethanesulfonate, [bmim][OTf]) were used as solvents instead of dimethyl formamide. The effect of the structure of anions and cations in ionic liquid, and water content on the Se reduction rate was investigated. In addition, the Se reduction pathway was also explored using UV-vis spectroscopy. The results show that the Se reduction rate is significantly increased by ∼1.4 times using [bmim][BF4], mainly by activating the water molecules.


 Please wait while we load your content...
Please wait while we load your content...