Catalyst-free ultrasonic-promoted multicomponent synthesis of tertiary α-amino phosphonates†
Abstract
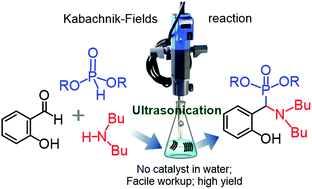
A highly efficient and green protocol has been developed for the synthesis of a new class of tertiary α-amino phosphonates (TAPs) via the Kabachnik–Fields reactions of substituted salicylaldehydes and 2-hydroxy naphthaldehyde, di-n-butylamine, and di-or tri-alkylphosphites in water under ultrasound irradiation as well as under conventional conditions. These multi-component reactions were performed at room temperature without using any catalysts in water. The TAPs were obtained in higher yields (84% to 94%) within shorter reaction times (6 min to 35 min) than those of conventional reactions after simple workup and purification procedures.


 Please wait while we load your content...
Please wait while we load your content...