IrO2–TiO2 electrocatalysts for the hydrogen evolution reaction in acidic water electrolysis without activation†
Abstract
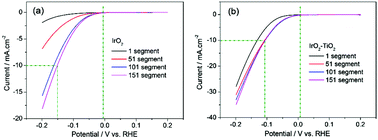
The development of highly active and long-term stable electrocatalysts for the hydrogen evolution reaction (HER) is very important. Because of the hysteresis phenomenon, IrO2 is rarely used as a cathode material for the HER. Herein, an IrO2–TiO2 composite oxide was prepared using the thermal decomposition method. The physical and electrochemical characterization of the materials was achieved by scanning electron microscopy (SEM), X-ray fluorescence (XRF), X-ray diffraction (XRD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). In the process of the HER, the current of IrO2 is only 1.91 mA cm−2@−0.2 V in the first segment scan. However, at the 51, 101 and 151 segment scan, the HER current increases to 6.85, 15.7 and 18.2 mA cm−2@−0.2 V, respectively. During the activation process of IrO2, the HER current has increased ten times. Compared with the HER activity of IrO2, there is almost no hysteresis for the IrO2–TiO2 electrode. In the first segment scan, the HER current has already reached 27.9 mA cm−2@−0.2 V and further increased to 31.1, 33.1 and 35.0 mA cm−2 at the 51, 101 and 151 segment scan. The difference between them is not significant, which means that the IrO2–TiO2 electrode does not need activation. The IrO2–TiO2 electrode has exhibited a higher HER activity than the IrO2 electrode, which may be attributed to the electronic structure modification and the increase of the electrochemical area.


 Please wait while we load your content...
Please wait while we load your content...