Luminescent closed shell nickel(ii) pyridyl-azo-oximates and the open shell anion radical congener: molecular and electronic structure, ligand redox behaviour and biological activity†
Abstract
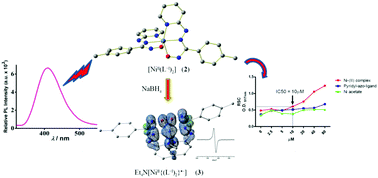
Luminescent nickel(II) complexes of type [NiII(L−I)2], 2, have been synthesized using photosensitizer redox-active oxime ligands HL, 1, incorporating the π-acidic azo and pyridyl functions. The redox non-innocent behaviour of the ligands has been exploited to isolate the open shell Ni(II)-bound azo-oxime anion radical complexes of type Et4N[NiII{(L−I)2}•−], 3, via reduction with NaBH4. The superior stabilization of the unpaired spin over the ligand framework has been established by EPR and DFT studies. The anti-bacterial activity of 2 has been scrutinized and it has been found to exhibit potential radical scavenging activity.


 Please wait while we load your content...
Please wait while we load your content...