Fluorination induced electronic effects on a Pt(ii) square-planar complex of the o-phenylenediimine ligand†
Abstract
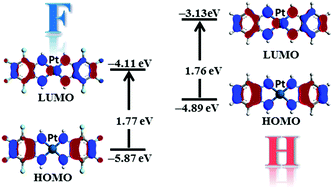
A novel complex [Pt(C6F4(NH)2)2] (2), formed by a Pt(II) cation bound to two molecules of the tetrafluorinated 1,2-phenylenediimine ligand, has been synthesized and characterized by electrochemical and XRD measurements, UV-vis-NIR spectroscopy as well as by DFT and TD-DFT calculations. The effect induced by the fluorine atoms has been highlighted by comparison with the corresponding Ni (1H) and Pt (2H) hydrogenated complexes. The cyclic voltammetry data show that the reduction and the oxidation processes of 2 are easier and more difficult (by about 0.5 V), respectively, compared to those of 1H and 2H, suggesting that the electron withdrawing ability of the fluorine atoms lowers the energy level of both the HOMO and the LUMO. UV-vis-NIR measurements are similar for all the three complexes, indicating similar HOMO–LUMO gaps and that the effects of fluorination on the frontier orbitals are roughly the same. Moreover, polymorphism in the powder form of 2 has been highlighted by XRD measurements while the film presents only one phase. Furthermore, this complex shows a field-effect for n-type carriers. All the experimental results are also supported by the calculations, which show the role played by the fluorine atoms in the electronic structure of 2.


 Please wait while we load your content...
Please wait while we load your content...