The mechanism of electropolymerization of nickel(ii) salen type complexes†
Abstract
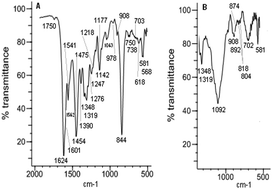
Ni(II) complexes with (±)-trans-N,N′-bis(salicylidene)-1,2-cyclohexanediamine ([Ni(salcn)]), and its methyl ([Ni(salcn(Me))]) and tert-butyl ([Ni(salcn(Bu))]) derivatives have been synthesized. Anodic electropolymerization has been used to form electrodes modified by polymer films. FTIR and FTIR ATR methods showed that the obtained films consist of polymers of phenyl–phenyl type. The structure of poly[Ni(salcn)] is built with 1,2,4- and 1,2,3,5-substituted chains. A three-step process of the oxidation of the complexes and their films has been ascertained on the basis of cyclic voltammetry measurements. Furthermore we have also detected the oxidized species which probably serve as intermediates for polymer formation. The influence of the substituent in the phenolate moiety on the type of reaction after which the dimerization and polymerization reaction occurs has been evidenced. Furthermore the substituent dependence on the stability of the phenoxyl radical complex and the Ni(II)-phenoxonium cation has been noticed. For the [Ni(salcn(Bu))] complex, an additional electropolymerization step – the adsorption of the reagent on the electrode surface – has been observed.


 Please wait while we load your content...
Please wait while we load your content...