Ligand and solvent effects in the formation and self-assembly of a metallosupramolecular cage†
Abstract
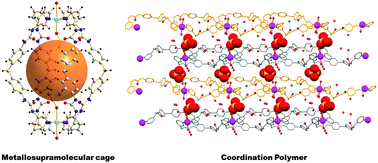
Two bis-pyridyl-bis-urea ligands namely N,N′-bis-(3-pyridyl)diphenylmethylene-bis-urea (L1) and N,N′-bis-(3-picolyl)diphenylmethylene-bis-urea (L2) have been reacted with a Cu(II) salt resulting in the formation of a metallosupramolecular cage [{Cu2(μ-L1)4(DMSO)2(H2O)2}·SO4·X] (1) and a one-dimensional coordination polymer [{Cu(1)(μ-L2)2(H2O)2}{Cu(2)(μ-L2)2(H2O)2}·2SO4·9H2O·X]n (2) (where DMSO = dimethylsulfoxide, and X = disordered lattice included solvent molecules), respectively. The single crystal structures of 1 and 2 are discussed in the context of the effect of the ligands, particularly the hydrogen bonding functionality of the ligand, on the supramolecular structural diversities observed in these metal organic compounds. The supramolecular packing of 1 is clearly influenced by the nature of the solvent and ligand used; mixtures of DMSO/MeOH or DMSO/H2O lead to the formation of blue crystals or a hydrogel, respectively.


 Please wait while we load your content...
Please wait while we load your content...