Synthesis and evaluation of benzothiazole-triazole and benzothiadiazole-triazole scaffolds as potential molecular probes for amyloid-β aggregation†
Abstract
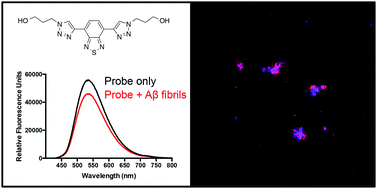
Small-molecule ligands that bind to misfolded protein aggregates are essential tools for the study and detection of pathological hallmarks in neurodegenerative disorders, such as Alzheimer's disease (AD). In the present study, three compounds (one benzothiazole-triazole, L1, and two benzothiadiazole-triazoles, L2 and L3) were synthesized via a modular approach (azide–alkyne cycloaddition) and evaluated as potential ligands for amyloid-β (Aβ) aggregates. The binding to amyloid-like fibrils, generated from recombinant Aβ1–42, were studied and the binding specificity to amyloid deposits was evaluated in brain sections from transgenic mice with AD pathology. All three derivatives showed significant reduced emission in the presence of recombinant Aβ1–42 amyloid fibrils. In addition, the observed binding to Aβ deposits in tissue sections suggests that the benzothiazole-triazole and benzothiadiazole-triazole structures are promising molecular scaffolds that can be modified for binding to specific protein aggregates.


 Please wait while we load your content...
Please wait while we load your content...