Pyrazolobenzothiazine-based carbothioamides as new structural leads for the inhibition of monoamine oxidases: design, synthesis, in vitro bioevaluation and molecular docking studies†‡
Abstract
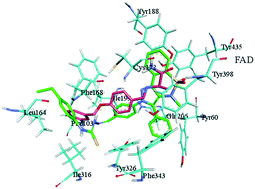
Two new series of pyrazolobenzothiazine-based carbothioamides (3a–o and 4a–o) were synthesized using saccharin as the starting material. The synthesized derivatives were investigated for their ability to inhibit monoamine oxidases (MAO). Compound 3b was found to be a very potent MAO-A inhibitor with an IC50 value of 0.003 ± 0.0007 μM, while compound 4d was the most effective inhibitor of MAO-B having an IC50 value of 0.02 ± 0.001 μM. Molecular docking studies were performed to identify the probable binding modes in the active site of the monoamine oxidase enzymes. The synthetic and computational investigations in the current work suggested that these newly identified inhibitors may serve as a powerful starting point for the exploration and optimization of potential therapeutic agents targeting Parkinson's disease.


 Please wait while we load your content...
Please wait while we load your content...