The DNA target determines the dimerization partner selected by bHLHZ-like hybrid proteins AhRJun and ArntFos†
Abstract
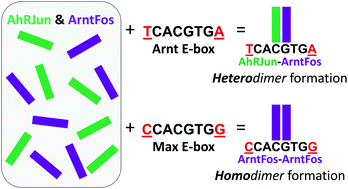
The molecular basis of protein–partner selection and DNA binding of the basic helix–loop–helix (bHLH) and basic region-leucine zipper (bZIP) superfamilies of dimeric transcription factors is fundamental toward understanding gene regulation. Because these families share structural similarities, we swapped the bHLH and leucine zipper (LZ) modules between families to uncover how individual modules influence protein-partnering and protein:DNA complexation. We previously described ArntFos, a bHLHZ-like hybrid of the bHLH domain from the bHLH/PAS protein Arnt and LZ from the bZIP protein c-Fos, binding to the Arnt E-box site (TCACGTGA) as a homodimer. Herein, we describe a heterodimer between ArntFos and AhRJun, a hybrid of the bHLH domain from AhR and LZ of JunD. We designed AhRJun and ArntFos to heterodimerize, given the strong interaction between native AhR/Arnt and Jun/Fos, but the hybrids showed no preference for hetero- or homo-dimerization in Y2H assays. However, adding a specific DNA target drove the formation of a single dimeric protein species over others. EMSA showed that the AhRJun/ArntFos heterodimer binds to the cognate DNA site XRE1 (TTGCGTG) with Kd = 337 nM. Unexpectedly, the palindromic Arnt E-box drove the binding of the AhRJun/ArntFos heterodimer (Kd = 276 nM)—not the ArntFos homodimer—that binds to the Arnt E-box. However, the dimerization preference switched to the ArntFos homodimer when the variant Max E-box (CCACGTGG) was used. We conclude that the DNA sites themselves are the primary determinants of dimerization specificity for AhRJun and ArntFos, not the JunD and c-Fos LZs, a result that sheds light on the dynamics of protein/protein and protein:DNA interactions and the structural modularity of bHLH and bZIP proteins.


 Please wait while we load your content...
Please wait while we load your content...