How RNase HI (Escherichia coli) promoted site-selective hydrolysis works on RNA in duplex with carba-LNA and LNA substituted antisense strands in an antisense strategy context?†
Abstract
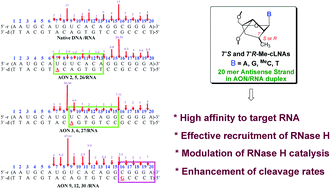
A detailed kinetic study of 36 single modified AON–RNA heteroduplexes shows that substitution of a single native nucleotide in the antisense strand (AON) by locked nucleic acid (LNA) or by diastereomerically pure carba-LNA results in site-dependent modulation of RNase H promoted cleavage of complementary mRNA strands by 2 to 5 fold at 5′-GpN-3′ cleavage sites, giving up to 70% of the RNA cleavage products. The experiments have been performed using RNase HI of Escherichia coli. The 2nd best cleavage site, being the 5′-ApN-3′ sites, cleaves up to 23%, depending upon the substitution site in 36 isosequential complementary AONs. A comparison of the modified AON promoted RNA cleavage rates with that of the native AON shows that sequence-specificity is considerably enhanced as a result of modification. Clearly, relatively weaker 5′-purine (Pu)–pyrimidine (Py)-3′ stacking in the complementary RNA strand is preferred (giving ∼90% of total cleavage products), which plays an important role in RNase H promoted RNA cleavage. A plausible mechanism of RNase H mediated cleavage of the RNA has been proposed to be two-fold, dictated by the balancing effect of the aromatic character of the purine aglycone: first, the locally formed 9-guanylate ion (pKa 9.3, ∼18–20% N1 ionized at pH 8) alters the adjoining sugar–phosphate backbone around the scissile phosphate, transforming its sugar N/S conformational equilibrium, to preferential S-type, causing preferential cleavage at 5′-GpN-3′ sites around the center of 20 mer complementary mRNA. Second, the weaker nearest-neighbor strength of 5′-Pu-p-Py-3′ stacking promotes preferential 5′-GpN-3′ and 5′-ApN-3′ cleavage, providing ∼90% of the total products, compared to ∼50% in that of the native one, because of the cLNA/LNA substituent effect on the neighboring 5′-Pu-p-Py-3′ sites, providing both local steric flexibility and additional hydration. This facilitates both the water and water/Mg2+ ion availability at the cleavage site causing sequence-specific hydrolysis of the phosphodiester bond of scissile phosphate. The enhancement of the total rate of cleavage of the complementary mRNA strand by up to 25%, presented in this work, provides opportunities to engineer a single modification site in appropriately substituted AONs to design an effective antisense strategy based on the nucleolytic stability of the AON strand versus RNase H capability to cleave the complementary RNA strand.


 Please wait while we load your content...
Please wait while we load your content...