Photochemical intramolecular amination for the synthesis of heterocycles†
Abstract
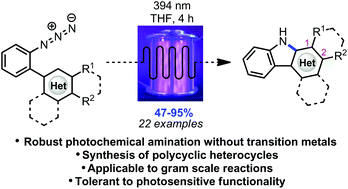
Polycyclic heterocycles can be formed in good to excellent yields via photochemical conversion of the corresponding substituted aryl azides under irradiation with purple LEDs in a continuous flow reactor. The experimental set-up is tolerant to UV-sensitive functional groups while affording diverse carbazoles, as well as an indole and pyrrole framework, in short reaction times. The photochemical method is presumed to progress through a mechanism differing from the other methods of azide activation involving transition metal catalysis.


 Please wait while we load your content...
Please wait while we load your content...