Metal-free oxidative cyclization of 2-aminobenzothiazoles and cyclic ketones enabled by the combination of elemental sulfur and oxygen†
Abstract
Metal-free oxidative cyclization for the one-pot synthesis of benzo[d]imidazo[2,1-b]thiazoles from 2-aminobenzothiazoles and cyclic ketones is described. Elemental sulfur combined with molecular oxygen as the benign co-oxidant was found to be unique and highly effective to promote this transformation without the aid of any metal salts. Various cyclic ketones smoothly reacted with 2-aminobenzothiazoles to give functional benzo[d]imidazo[2,1-b]thiazoles in good to very high yields, which thereby demonstrated the synthetic convergence of this methodology.
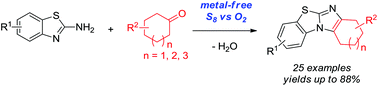


 Please wait while we load your content...
Please wait while we load your content...