Synthesis of 2-substituted quinazolines by CsOH-mediated direct aerobic oxidative cyclocondensation of 2-aminoarylmethanols with nitriles in air†
Abstract
By using air as the superior oxidant, a highly atom-efficient synthesis of 2-substituted quinazolines is developed by a CsOH-mediated direct aerobic oxidative reaction of the readily available and stable 2-aminoarylmethanols and nitriles. Effectively working as the promoter in the alcohol oxidation, nitrile hydration, and cyclocondensation steps, CsOH is the best base for the reaction. A similar method can also be extended to the synthesis of substituted quinolines starting from methyl ketones instead of nitriles.
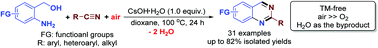


 Please wait while we load your content...
Please wait while we load your content...