Efficient synthesis of isoquinolines in water by a Pd-catalyzed tandem reaction of functionalized alkylnitriles with arylboronic acids†
Abstract
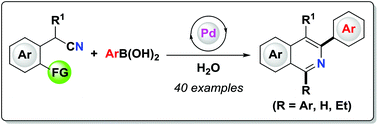
A palladium-catalyzed tandem reaction of 2-(cyanomethyl)benzonitriles or 2-(2-carbonylphenyl)acetonitriles with arylboronic acids in water has been developed for the first time. This reaction features good functional group tolerance and provides a new strategy for the synthesis of diverse isoquinolines under mild conditions. The use of water as the reaction medium makes the synthesis process environmentally benign. Preliminary mechanistic experiments indicate that the major reaction pathway involves carbopalladation of the C(sp3)–cyano group and subsequent intramolecular cyclization findings that were further supported by density functional theory (DFT) calculations.


 Please wait while we load your content...
Please wait while we load your content...