Biocatalytic access to nonracemic γ-oxo esters via stereoselective reduction using ene-reductases†
Abstract
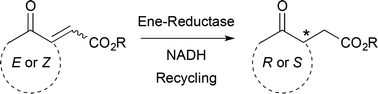
The asymmetric bioreduction of α,β-unsaturated γ-keto esters using ene-reductases from the Old Yellow Enzyme family proceeds with excellent stereoselectivity and high conversion levels, covering a broad range of acyclic and cyclic derivatives. Various strategies were employed to provide access to both enantiomers, which are versatile precursors of bioactive molecules. The regioselectivity of hydride addition on di-activated alkenes was elucidated by isotopic labeling experiments and showed strong preference for the keto moiety as activating/binding group as opposed to the ester. Finally, chemoenzymatic synthesis of (R)-2-(2-oxocyclohexyl)acetic acid was achieved in high ee on a preparative scale combining enzymatic reduction followed by ester hydrogenolysis.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis


 Please wait while we load your content...
Please wait while we load your content...