Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries†
Abstract
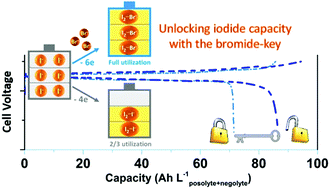
Highly soluble iodide/triiodide (I−/I3−) couples are one of the most promising redox-active species for high-energy-density electrochemical energy storage applications. However, to ensure high reversibility, only two-thirds of the iodide capacity is accessed and one-third of the iodide ions act as a complexing agent to stabilize the iodine (I2), forming I3− (I2I−). Here, we exploit bromide ions (Br−) as a complexing agent to stabilize the iodine, forming iodine–bromide ions (I2Br−), which frees up iodide ions and increases the capacity. Applying this strategy, we demonstrate a novel zinc/iodine–bromide battery to achieve an energy density of 101 W h Lposolyte+negolyte−1 (or 202 W h Lposolyte−1), which is the highest energy density achieved for aqueous flow batteries to date. This strategy can be further generalized to nonaqueous iodide-based batteries (i.e. lithium/polyiodide battery), offering new opportunities to improve high-energy iodide-based energy storage technologies.


 Please wait while we load your content...
Please wait while we load your content...