Inter-cluster distance dependence of electrical properties in single crystals of a mixed-valence polyoxometalate†
Abstract
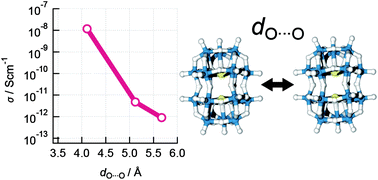
The electrical conductivity of mixed-valence [MoV2MoVI16O54(SO3)2]6− tetraalkylammonium salts was investigated through dependence on the inter-cluster distance that is controlled by tetraethylammonium, tetrapropylammonium, and tetrabutylammonium cations. The crystallographic analysis of single crystals revealed that the inter-cluster distances are dependent on the chain length of the alkyl groups on the counter cations. In addition, the electrical conductivities of the single crystals were found to be dependent on both temperature and chain length. Mixed-valence polyoxometalate (POM) clusters are considered to be a molecular particle of Mo bronze by which highly ordered networks will be developed using single crystals, where POMs are rather small and have a well-organized structure compared to colloidal nanostructures.


 Please wait while we load your content...
Please wait while we load your content...