A highly stable l-alanine-based mono(aquated) Mn(ii) complex as a T1-weighted MRI contrast agent†
Abstract
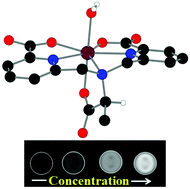
The synthesized lithium (S)-6,6′-(1-carboxyethylazanediyl)bis(methylene)dipicolinate (Li3cbda) is a new chiral, alanine-based ligand bearing two picolinate functionalities. The trianionic form of the ligand [(cbda)3−] constitutes a seven-coordinate, water-soluble, pentagonal bipyramidal Mn(II) complex (1). The structural analysis reveals the presence of a water coordinating site in the complex. The complex is thermodynamically very stable, and the stability is not affected by the presence of physiological anions (HCO3−, PO43−, and F−). The pH of the medium exerts a small effect on the stability of the complex. The r1 relaxivity of 3.02 mM−1 s−1 is exhibited by the complex at 1.41 T, pH ∼7.4, and 25 °C. Phantom images obtained via a clinical MRI BRIVO MR355 system established concentration-dependent signal enhancement by the complex. The cytotoxicity test confirmed complex 1 as a biocompatible potential T1-weighted MRI contrast agent.


 Please wait while we load your content...
Please wait while we load your content...