Enhanced Congo red dye removal from aqueous solutions using iron nanoparticles: adsorption, kinetics, and equilibrium studies†
Abstract
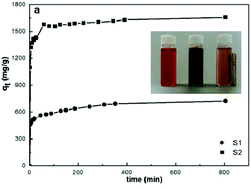
We report on the Congo red dye removal properties of body centred cubic and amorphous iron nanoparticles, synthesized by a facile borohydride reduction method under ambient conditions. We have analyzed the adsorption of Congo red as a function of dye concentration, time, and temperature and measured a Congo red adsorption capacity of 1735 mg g−1 for the amorphous iron nanoparticles. To our knowledge, this is the highest value reported so far for Congo red adsorption. The acquired data have been evaluated applying various models for adsorption kinetics and thermodynamic studies. The isotherm models as well as acquired Fourier transform infrared spectra suggest that both chemi- and physisorption occur for Congo red adsorption on iron nanoparticles, where chemisorption appears to be dominant. The kinetics of adsorption of Congo red on both bcc-structured and amorphous iron follow a pseudo-second order equation and are characterized by high initial adsorption rates. Diffusion studies indicate that adsorption occurs in two stages, namely film diffusion followed by intraparticle diffusion. Our studies show that amorphous iron nanoparticles are highly promising for dye adsorption and wastewater treatment applications.


 Please wait while we load your content...
Please wait while we load your content...