Selective hydrosilylation of alkynes and ketones: contrasting reactivity between cationic 3-iminophosphine palladium and nickel complexes†
Abstract
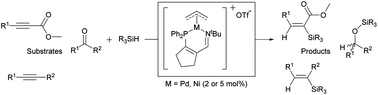
The catalytic hydrosilylation of alkynes and ketones has been explored utilizing palladium- and nickel(allyl) complexes supported by 3-iminophosphine ligands. Palladium and nickel demonstrated distinctly different reactivity profiles, with palladium proving very effective for the hydrosilylation of electron-deficient alkynes, while nickel excelled with ketones and internal alkynes. Additionally, in many cases, regioselective hydrosilylation was observed.


 Please wait while we load your content...
Please wait while we load your content...