Bis-cyclometalated iridium complexes with electronically modified aryl isocyanide ancillary ligands†
Abstract
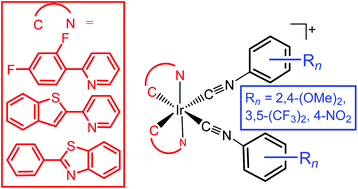
In this work we report a study on the effect of systematic ancillary ligand modifications on electrochemical and photophysical properties of cationic biscyclometalated bis(arylisocyanide)iridium(III) complexes. Nine new Ir(III) complexes were synthesized using three different cyclometalating (C^N) ligands (2,4-difluorophenylpyridine (F2ppy), 2-benzothienylpyridine (btp), and 2-phenylbenzothiazole (bt)) with three aryl isocyanide ancillary ligands (2,4-dimethoxyphenyl isocyanide (CNArOMe), 3,5-bis(trifluoromethyl)phenyl isocyanide (CNArCF3) and 4-nitrophenyl isocyanide (CNArNO2)). Systematic modifications of ancillary ligands with electron-donating or electron-withdrawing groups have a very minor influence on the positions of the absorption and emission bands, suggesting that aryl isocyanide ancillary ligands minimally perturb the primarily ligand-centered emissive states, but still can control the dynamics of the excited state. Replacing electron-donating groups with electron-withdrawing group influences kr and/or knr, resulting in changes in the lifetimes and quantum yields. In addition, we reveal that electronic structures can be substantially altered by incorporating electron-donating or electron-withdrawing groups on the aryl isocyanide ancillary ligand, with different magnitudes of the perturbation depending on the cyclometalating C^N ligand. Particularly, the formally IrIV/IrIII oxidation couple can be perturbed by over 200 mV when electron-donating substituents are replaced with electron-withdrawing groups.


 Please wait while we load your content...
Please wait while we load your content...