Control of the reversibility during boronic ester formation: application to the construction of ferrocene dimers and trimers†
Abstract
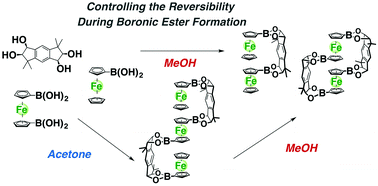
Control of the reversibility during boronic ester formation from boronic acids and diols was found to be possible by choosing an appropriate solvent. As an example, ferrocene dimers and trimers were constructed by using tetrol 1 with an indacene framework, 1,1′-ferrocenediboronic acid 2, and ferrocenemonoboronic acid 4. When equimolar amounts of 1 and 2 were mixed in methanol under equilibrating conditions, two kinds of stacked ferrocene dimers homo- and hetero-3 were selectively obtained depending on the reaction time and both structures were determined by X-ray crystallographic analysis. On the other hand, the ferrocene trimer 7 was successfully constructed by stepwise assembly in the presence of anhydrous magnesium sulfate in acetone where the equilibration of boronic esters was suppressed, while no formation of ferrocene trimer 7 was detected when all components 1, 2 and 4 (2 : 1 : 2 ratio) for trimer 7 were mixed at a time in methanol under equilibrating conditions.


 Please wait while we load your content...
Please wait while we load your content...