Aryl-NHC-group 13 trimethyl complexes: structural, stability and bonding insights†
Abstract
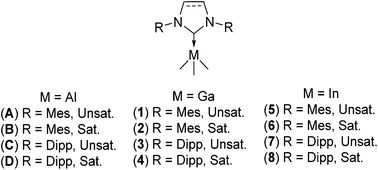
Treatment of aromatic N-substituted N-heterocyclic carbenes (NHCs) with trimethyl-gallium and -indium yielded the new Lewis acid–base adducts, IMes·GaMe3 (1), SIMes·GaMe3 (2), IPr·GaMe3 (3), SIPr·GaMe3 (4), IMes·InMe3 (5), SIMes·InMe3 (6), IPr·InMe3 (7), and SIPr·InMe3 (8), with all complexes being identified by X-ray diffraction, IR, and multinuclear NMR analyses. Complex stability was found to be largely dependent on the nature of the constituent NHC ligands. Percent buried volume (%VBur) and topographic steric map analyses were employed to quantify and elucidate the observed trends. Additionally, a detailed bond snapping energy (BSE) decomposition analysis focusing on both steric and orbital interactions of the M–NHC bond (M = Al, Ga and In) has been performed.


 Please wait while we load your content...
Please wait while we load your content...