Bipyridine based metallogels: an unprecedented difference in photochemical and chemical reduction in the in situ nanoparticle formation†
Abstract
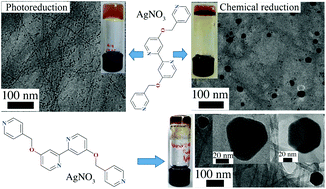
Metal co-ordination induced supramolecular gelation of low molecular weight organic ligands is a rapidly expanding area of research due to the potential in creating hierarchically self-assembled multi-stimuli responsive materials. In this context, structurally simple O-methylpyridine derivatives of 4,4′-dihydroxy-2,2′-bipyridine ligands are reported. Upon complexation with Ag(I) ions in aqueous dimethyl sulfoxide (DMSO) solutions the ligands spontaneously form metallosupramolecular gels at concentrations as low as 0.6 w/v%. The metal ions induce the self-assembly of three dimensional (3D) fibrillar networks followed by the spontaneous in situ reduction of the Ag-centers to silver nanoparticles (AgNPs) when exposed to daylight. Significant size and morphological differences of the AgNP's was observed between the standard chemical and photochemical reduction of the metallogels. The gelation ability, the nanoparticle formation and rheological properties were found to be depend on the ligand structure, while the strength of the gels is affected by the water content of the gels.


 Please wait while we load your content...
Please wait while we load your content...