The mechanism of selective catalytic reduction of NOx on Cu-SSZ-13 – a computational study†
Abstract
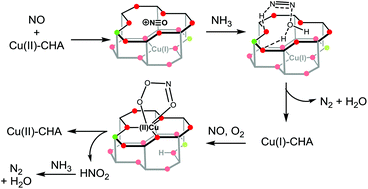
The copper-exchanged aluminosilicate zeolite SSZ-13 is a leading catalyst for the selective catalytic reduction of NO. Density functional theory calculations are used to construct a complete catalytic cycle of this process paying special attention to the coordination geometries and redox states of copper. N2 can be produced in the reduction half-cycle via a nitrosamine intermediate generated from the reaction of the additive reductant NH3 with a NO+ intermediate stabilized by the zeolite lattice. The decomposition of this nitrosamine species can be assisted by incipient Brønsted acid sites generated during catalysis. Our calculations also suggest that the reoxidation of Cu(I) to Cu(II) requires the addition of both NO and O2. The production of a second equivalent of N2 during the oxidation half-cycle proceeds through a peroxynitrite intermediate to form a Cu–nitrite intermediate, which may react with an acid, either HNO2 or NH4+ to close the catalytic cycle. Models of copper neutralized by an external hydroxide ligand are also examined. These calculations form a key basis for understanding the mechanism of NO reduction in Cu-SSZ-13 in order to develop strategies for rationally optimizing the performance in future experiments.

 Please wait while we load your content...
Please wait while we load your content...