Unravelling the mechanisms of CO2 hydrogenation to methanol on Cu-based catalysts using first-principles multiscale modelling and experiments†
Abstract
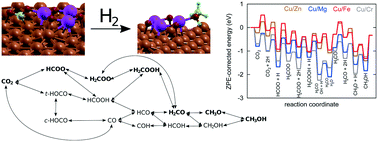
A way to address the challenge of carbon dioxide emissions causing global warming and climate change is the heterogeneous catalytic hydrogenation of CO2. When surplus electrical energy is available, hydrogen may be produced and used for conversion of CO2 into fuels and chemicals. Industrially, a multifunctional metallic copper and zinc oxide catalyst on alumina (CZA) is commonly used. Presently, first-principles multiscale modelling was accomplished for a commercial-like catalyst (Zn3O3/Cu) and three other Cu/metal oxide combinations (Cr3O3/Cu, Fe3O3/Cu, and Mg3O3/Cu), synthesised via co-precipitation, characterised and experimentally tested. Ab initio plane wave density functional theory calculations were performed using different structural models to elucidate the adsorption of intermediates and elementary reaction steps, considering thermodynamics and kinetics. The results were fed into mean-field micro-kinetic expressions to calculate the conversion and selectivity in a continuous flow stirred-tank reactor vessel for various temperatures and pressures. Kinetic Monte Carlo simulations were used to obtain the detailed surface coverage, turnover frequency and catalytic performance. Although no experimental input besides the structure was applied for physico-chemical mechanism description, measurements were in agreement with theoretical predictions. It was shown that the formate species pathway (HCOO → H2COO → H2COOH → H2CO → H3CO) predominates on the examined Cu-based catalysts, although there are variations in the rate determining steps and the most abundant surface intermediate fractions. Whereas Zn3O3/Cu exhibited the highest conversion and a moderate CH3OH product selectivity, the former was lower for Mg3O3/Cu. Interestingly, Cr3O3/Cu was optimal in terms of yield, but with extremely low CH3OH productivity, while Fe3O3/Cu performed poorly overall. Our results highlight the superiority of Zn3O3/Cu, combining the estimations on micro-, meso- and reactor operation scales. Insights beyond traditional activity experiments may be attained and employed in full-scale reactor engineering and optimisation.


 Please wait while we load your content...
Please wait while we load your content...