Efficient acceptorless dehydrogenation of secondary alcohols to ketones mediated by a PNN-Ru(ii) catalyst†
Abstract
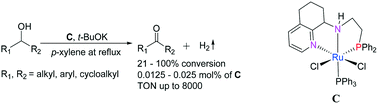
Four types of ruthenium(II) complexes, [fac-PNN]RuH(PPh3)(CO) (A), [fac-PNHN]RuH(η1-BH4)(CO) (B), [fac-PNHN]RuCl2(PPh3) (C) and [fac-PNHN]RuH(η1-BH4)(PPh3) (D) (where PNHN and PNN are N-(2-(diphenylphosphino)ethyl)-5,6,7,8-tetrahydroquinoline-8-amine and its deprotonated derivative), have been synthesized and assessed as catalysts for the acceptorless dehydrogenation of secondary alcohols to afford ketones. It was found that C, in combination with t-BuOK, proved the most effective and versatile catalyst allowing aromatic-, aliphatic- and cycloalkyl-containing alcohols to be efficiently converted to their corresponding ketones with particularly high values of TON achievable. Furthermore, the mechanism for this PNN-Ru mediated process been proposed on the basis of a number of intermediates that have been characterized by EI-MS and NMR spectroscopy. These catalysts show great potential for applications in atom-economic synthesis as well as in the development of organic hydride-based hydrogen storage systems.


 Please wait while we load your content...
Please wait while we load your content...