Identification of sulfate species and their influence on SCR performance of Cu/CHA catalyst†
Abstract
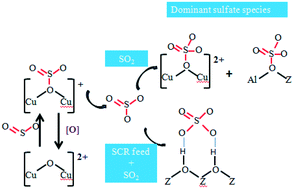
An investigation into sulfate formation on Cu/CHA zeolites was conducted to confirm the main sulfate species that deactivate the catalyst. SO2-TPD and controlled regeneration of poisoned catalysts were carried out to study the physicochemical properties of the sulfates formed under different conditions. The results revealed that three sulfate species existed on the catalyst surface, namely H2SO4, CuSO4 and Al2(SO4)3. H2O and NH3-SCR feed could improve the formation of H2SO4via an increase in the reaction rate of SOx with protons and decrease the thermal stability of all sulfates via the protons' strong polarization effect. The poisoning effect of ammonium sulfate was more emphasized during sulfation in the presence of simulated exhaust gas. Additionally, in contrast to Cu/SSZ-13, Cu/SAPO-34 favored the formation of stable Al2(SO4)3 species, which resulted in unrecoverable SCR activity in an inert atmosphere below 650 °C.


 Please wait while we load your content...
Please wait while we load your content...