Photocatalytic coupling of formaldehyde to ethylene glycol and glycolaldehyde over bismuth vanadate with controllable facets and cocatalysts†
Abstract
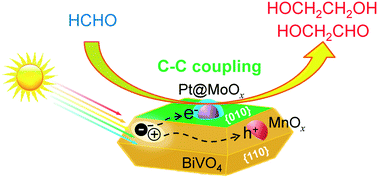
Bismuth vanadate (BiVO4) single crystals with controllable facets and cocatalysts were synthesized and studied for photocatalytic coupling of formaldehyde into C2 compounds mainly including ethylene glycol and glycolaldehyde. By using chloride anions as a morphology-controlling agent, we succeeded in synthesizing BiVO4 single crystals with a uniform truncated tetragonal bipyramidal morphology enclosed with {010} and {110} facets. The ratio of exposed {010} and {110} facets could be regulated by changing the concentration of Cl−. BiVO4 with an equal fraction of exposed {010} and {110} facets exhibited the highest capability of electron–hole separation and the highest C2-compound yield. The loading of core–shell structured Pt@MoOx and MnOx particles onto {010} and {110} facets, respectively, further enhanced the formation of C2 compounds. Our studies suggested that the Pt core and the MnOx particles accelerated the separation of photogenerated electron–hole pairs, whereas the MoOx shell catalyzed the coupling of HCHO possibly via a redox mechanism. The yields of C2 compounds and ethylene glycol reached 21% and 11%, respectively, under irradiation with UV-vis light for 12 h. Quantum yields of 11% and 4.8% were achieved for the coupling products under ultraviolet (350 nm) and visible (450 nm) light irradiation, respectively.


 Please wait while we load your content...
Please wait while we load your content...