Beyond sulfide-centric catalysis: recent advances in the catalytic cyclization reactions of sulfur ylides
Abstract
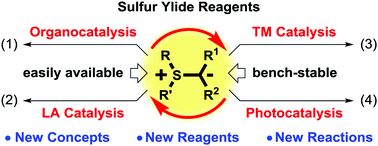
Great achievements in the asymmetric cyclization reactions of sulfur ylides have been reached by using chiral sulfides; however, this method usually suffers from the high loading of chiral sulfides. Over the past decade, new catalysis technologies beyond chiral sulfide-based catalysis have been gradually applied to the cyclizations of sulfur ylides. These technologies, including organocatalysis, organometallic catalysis and photocatalysis, can avoid the use of stoichiometric chiral pools and enable the development of new cyclization reactions of sulfur ylides. In this tutorial review, recent advances in this rapidly developing field will be highlighted with particular emphases on the catalytic mechanism and the development of new reactions, new reagents and new concepts.


 Please wait while we load your content...
Please wait while we load your content...