Rate constant for the H˙ + H2O → ˙OH + H2 reaction at elevated temperatures measured by pulse radiolysis
Abstract
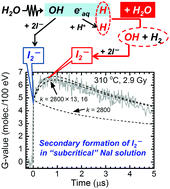
Maintaining the structural integrity of materials in nuclear power plants is an essential issue associated with safe operation. Hydrogen (H2) addition or injection to coolants is a powerful technique that has been widely applied such that the reducing conditions in the coolant water avoid corrosion and stress corrosion cracking (SCC). Because the radiation-induced reaction of ˙OH + H2 → H˙ + H2O plays a crucial role in these systems, the rate constant has been measured at operation temperatures of the reactors (285–300 °C) by pulse radiolysis, generating sufficient data for analysis. The reverse reaction H˙ + H2O → ˙OH + H2 is negligibly slow at ambient temperature; however, it accelerates considerably quickly at elevated temperatures. Although the reverse reaction reduces the effectiveness of H2 addition, reliable rate constants have not yet been measured. In this study, the rate constants have been determined in a temperature range of 250–350 °C by pulse radiolysis in an aqueous I− solution.


 Please wait while we load your content...
Please wait while we load your content...