Thermodynamic assessment of the oxygen reduction activity in aqueous solutions†
Abstract
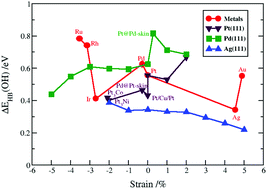
In the conventional theoretical approach, oxygen reduction reaction activities are assessed through a volcano plot using activity descriptors. The volcano plot relies on several approximations, e.g. the reaction kinetics are commonly overlooked and the interaction of hydrophilic intermediates with water is considered constant regardless of the metal surface. Herein, we demonstrate by means of density functional theory calculations that the binding energies of hydrophilic intermediates are strongly influenced by hydrogen bonding (HB) to surface water molecules. We find the HB energies of adsorbed OOH and OH on a number of active metallic (strained and non-strained Pt, Pd, Ag) and bimetallic (Pt3Ni, Pt3Co, PtCu, Pd@Pt-skin and Pt@Pd-skin) 111 surfaces to vary by up to 0.5 eV in energy. Furthermore, we show that the existence of a universal scaling line is a relative notion, contingent on how large errors in activity predictions can be tolerated. Scaling errors can be reduced substantially by partitioning data into subsets depending on the element comprising the surface layer. Finally, the activity volcano that explicitly includes HB and van der Waals interactions reproduces the right experimental trend for Pt and its alloys, but at the same time predicts Ag to be a more active catalyst than Pt. The latter result can be explained by having a fundamentally different water structure on Ag(111) than on the other metals, and the fact that reaction kinetics have been neglected in the analysis.


 Please wait while we load your content...
Please wait while we load your content...