The promoting effect of tetravalent cerium on the oxygen evolution activity of copper oxide catalysts†
Abstract
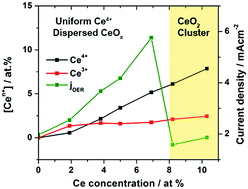
A new catalyst is presented for the oxygen evolution reaction (OER) based on cerium-modified copper oxide (CuOx) prepared using a facile electrodeposition procedure. Incorporation of Ce into CuOx leads to greatly improved OER activity, which reached an optimal value at a surface concentration of 6.9 at% Ce. Specifically, the OER current density at 400 mV overpotential for the most active Ce-modified CuOx catalyst (6.9 at% Ce) was 3.3 times greater compared to the pure CuOx. Coincident with the improved OER activity, Ce incorporation also leads to significant structural changes that manifested in increasing degrees of disorder. A further increase in the Ce concentration led to a decrease in the OER performance which can be attributed to the formation of a segregated CeO2 phase. A strong correlation was observed between the OER performance and tetravalent Ce (Ce4+) ion concentration, up to a concentration corresponding to CeO2 phase segregation. No particular trend was observed for the OER activity of these Ce-modified CuOx catalysts with respect to the surface concentration of Cu ions, surface oxygen species or catalyst structure. The stability of these CuOx catalysts at 5 mA cm−2 was also improved with Ce incorporation, and the overpotential required to sustain this current density is much lower than that of pure CuOx. Overall, this study provides new insights regarding the promoting effect of tetravalent Ce ions on the OER activity of CuOx-based OER catalysts in alkaline electrolytes.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...