On the elusive nature of oxygen binding at coordinatively unsaturated 3d transition metal centers in metal–organic frameworks†
Abstract
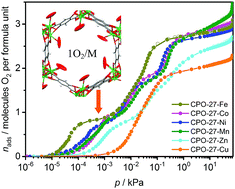
Using gas sorption measurements at ambient temperatures and in situ neutron powder diffraction methods, we have studied the interaction strengths and coordination geometries of O2 and N2 near the non-occupied coordination site (open metal site) in the isostructural MOF structures of the CPO-27-M/M-MOF-74 series (with M = Co, Ni, Mn and Cu). Our experimental observations are compared to periodic quantum chemical model calculations. Contrary to recent computational studies, our results, both experimental and theoretical, unequivocally suggest rather weak interactions between the M(II) coordinatively unsaturated centers and the adsorbate molecules, being mainly dispersive and electrostatic in nature. As a consequence, they exclude significant orbital charge transfer effects that could lead to superoxide/peroxide formation. Calculated binding energies appear in good agreement with the measured isosteric heats of adsorption in the range of 10–20 kJ mol−1. These, relatively weak host–guest interactions, lead to a tilted end-on geometry in all of the investigated M(II)–guest molecule adducts.


 Please wait while we load your content...
Please wait while we load your content...