The magnetic and adsorption properties of ZnO1−xSx nanoparticles†
Abstract
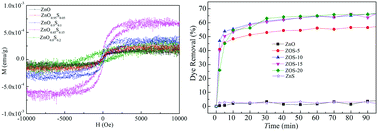
Sulfur is easy to be incorporated into ZnO nanoparticles by the solution-combustion method. Herein, the magnetic and adsorption properties of a series of ZnO1−xSx (x = 0, 0.05, 0.1, 0.15, and 0.2) nanoparticles were systematically investigated. The X-ray diffraction patterns show that the as-prepared ZnO1−xSx nanoparticles have the hexagonal wurtzite structure of ZnO with a low sulfur content that gradually transforms into the zinc blende structure of ZnS when the x value is greater than 0.1. PL spectra show several bands due to different transitions, which have been explained by the recombination of free excitons or defect-induced transitions. The introduction of sulfur not only modifies the bandgap of ZnO, but also impacts the concentration of Zn vacancies. The as-prepared ZnO shows weak room-temperature ferromagnetism, and the incorporation of sulfur improves the ferromagnetism owing to the increased concentration of Zn vacancies, which may be stabilized by the doped sulfur ions. The adsorption capability of ZnO1−xSx nanoparticles has been significantly improved, and the process can be well described by the pseudo-first-order kinetic model and the Freundlich isotherm model. The mechanism has been confirmed to be due to the active sulfate groups existing in zinc oxysulfide nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...