Charge-transfer and impulsive electronic-to-vibrational energy conversion in ferricyanide: ultrafast photoelectron and transient infrared studies†
Abstract
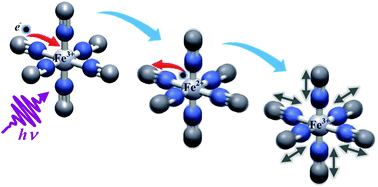
The photophysics of ferricyanide in H2O, D2O and ethylene glycol was studied upon excitation of ligand-to-metal charge transfer (LMCT) transitions by combining ultrafast photoelectron spectroscopy (PES) of liquids and transient vibrational spectroscopy. Upon 400 nm excitation in water, the PES results show a prompt reduction of the Fe3+ to Fe2+ and a back electron transfer in ∼0.5 ps concomitant with the appearance and decay of a strongly broadened infrared absorption at ∼2065 cm−1. In ethylene glycol, the same IR absorption band decays in ∼1 ps, implying a strong dependence of the back electron transfer on the solvent. Thereafter, the ground state ferric species is left vibrationally hot with significant excitation of up to two quanta of the CN-stretch modes, which completely decay on a 10 ps time scale. Under 265 nm excitation even higher CN-stretch levels are populated. Finally, from a tiny residual transient IR signal, we deduce that less than 2% of the excited species undergo photoaquation, in line with early flash photolysis experiments. The latter is more significant at 265 nm compared to 400 nm excitation, which suggests photodissociation in this system is an unlikely statistical process related to the large excess of vibrational energy.
- This article is part of the themed collections: Celebrating our 2019 Prize and Award winners and 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...