Size dependent behavior of Fe3O4 crystals during electrochemical (de)lithiation: an in situ X-ray diffraction, ex situ X-ray absorption spectroscopy, transmission electron microscopy and theoretical investigation†
Abstract
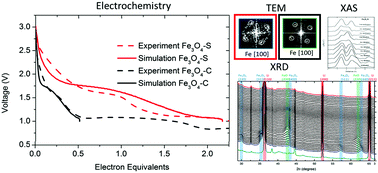
The iron oxide magnetite, Fe3O4, is a promising conversion type lithium ion battery anode material due to its high natural abundance, low cost and high theoretical capacity. While the close packing of ions in the inverse spinel structure of Fe3O4 enables high energy density, it also limits the kinetics of lithium ion diffusion in the material. Nanosizing of Fe3O4 to reduce the diffusion path length is an effective strategy for overcoming this issue and results in improved rate capability. However, the impact of nanosizing on the multiple structural transformations that occur during the electrochemical (de)lithiation reaction in Fe3O4 is poorly understood. In this study, the influence of crystallite size on the lithiation-conversion mechanisms in Fe3O4 is investigated using complementary X-ray techniques along with transmission electron microscopy (TEM) and continuum level simulations on electrodes of two different Fe3O4 crystallite sizes. In situ X-ray diffraction (XRD) measurements were utilized to track the changes to the crystalline phases during (de)lithiation. X-ray absorption spectroscopy (XAS) measurements at multiple points during the (de)lithiation processes provided local electronic and atomic structural information. Tracking the crystalline and nanocrystalline phases during the first (de)lithiation provides experimental evidence that (1) the lithiation mechanism is non-uniform and dependent on crystallite size, where increased Li+ diffusion length in larger crystals results in conversion to Fe0 metal while insertion of Li+ into spinel-Fe3O4 is still occurring, and (2) the disorder and size of the Fe metal domains formed when either material is fully lithiated impacts the homogeneity of the FeO phase formed during the subsequent delithiation.


 Please wait while we load your content...
Please wait while we load your content...