Molecular dynamics simulation of the local concentration and structure in multicomponent aerosol nanoparticles under atmospheric conditions†
Abstract
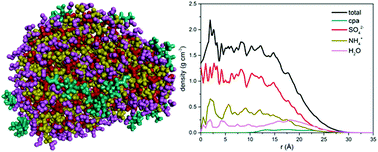
Molecular dynamics (MD) simulations were employed to investigate the local structure and local concentration in atmospheric nanoparticles consisting of an organic compound (cis-pinonic acid or n-C30H62), sulfate and ammonium ions, and water. Simulations in the isothermal–isobaric (NPT) statistical ensemble under atmospheric conditions with a prespecified number of molecules of the abovementioned compounds led to the formation of a nanoparticle. Calculations of the density profiles of all the chemical species in the nanoparticle, the corresponding radial pair distribution functions, and their mobility inside the nanoparticle revealed strong interactions developing between sulfate and ammonium ions. However, sulfate and ammonium ions prefer to populate the central part of the nanoparticle under the simulated conditions, whereas organic molecules like to reside at its outer surface. Sulfate and ammonium ions were practically immobile; in contrast, the organic molecules exhibited appreciable mobility at the outer surface of the nanoparticle. When the organic compound was a normal alkane (e.g. n-C30H62), a well-organized (crystalline-like) phase was rapidly formed at the free surface of the nanoparticle and remained separate from the rest of the species.


 Please wait while we load your content...
Please wait while we load your content...