Laser desorption single-conformation UV and IR spectroscopy of the sulfonamide drug sulfanilamide, the sulfanilamide–water complex, and the sulfanilamide dimer†
Abstract
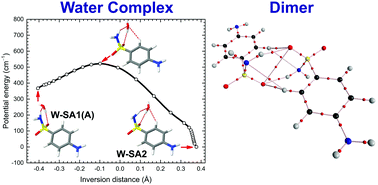
We have studied the conformational preferences of the sulfonamide drug sulfanilamide, its dimer, and its monohydrated complex through laser desorption single-conformation UV and IR spectroscopy in a molecular beam. Based on potential energy curves for the inversion of the anilinic and the sulfonamide NH2 groups calculated at DFT level, we suggest that the zero-point level wave function of the sulfanilamide monomer is appreciably delocalized over all four conformer wells. The sulfanilamide dimer, and the monohydrated complex each exhibit a single isomer in the molecular beam. The isomeric structures of the sulfanilamide dimer and the monohydrated sulfanilamide complex were assigned based on their conformer-specific IR spectra in the NH and OH stretch region. Quantum Theory of Atoms in Molecules (QTAIM) analysis of the calculated electron density in the water complex suggests that the water molecule is bound side-on in a hydrogen bonding pocket, donating one O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bond and accepting two hydrogen bonds, a NH⋯O and a CH⋯O hydrogen bond. QTAIM analysis of the dimer electron density suggests that the Ci symmetry dimer structure exhibits two dominating N–H⋯O
S hydrogen bond and accepting two hydrogen bonds, a NH⋯O and a CH⋯O hydrogen bond. QTAIM analysis of the dimer electron density suggests that the Ci symmetry dimer structure exhibits two dominating N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bonds, and three weaker types of interactions: two CH⋯O bonds, two CH⋯N bonds, and a chalcogen O⋯O interaction. Most interestingly, the molecular beam dimer structure closely resembles the R22 dimer unit – the dimer unit with the greatest interaction energy – of the α, γ, and δ crystal polymorphs. Interacting Quantum Atoms analysis provides evidence that the total intermolecular interaction in the dimer is dominated by the short-range exchange–correlation contribution.
S hydrogen bonds, and three weaker types of interactions: two CH⋯O bonds, two CH⋯N bonds, and a chalcogen O⋯O interaction. Most interestingly, the molecular beam dimer structure closely resembles the R22 dimer unit – the dimer unit with the greatest interaction energy – of the α, γ, and δ crystal polymorphs. Interacting Quantum Atoms analysis provides evidence that the total intermolecular interaction in the dimer is dominated by the short-range exchange–correlation contribution.


 Please wait while we load your content...
Please wait while we load your content...