Observing the real time formation of phosphine-ligated gold clusters by electrospray ionization mass spectrometry†
Abstract
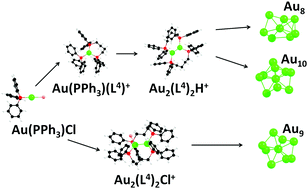
The early stages of reduction and nucleation of ligated gold clusters in solution are largely unknown due, in part, to high reaction rates and the inherent complexity of the process. This study demonstrates that the addition of a diphosphine ligand, 1–4-bis(diphenylphosphino)butane (L4) to a methanolic solution of the gold precursor, chloro(triphenylphosphine)gold(I) (Au(PPh3)Cl), results in the initial formation of organometallic complexes of the type [Au(L4)x(L4O)y(PPh3)z]+. These initial complexes lower the rate of gold reduction so that the reaction can be directly monitored over time from 1 min to over an hour using on-line electrospray ionization mass spectrometry (ESI-MS). The results indicate that the formation of cationic Au8(L4)42+, Au9(L4)4H2+ and Au10(L4)52+ clusters occurs through specific reaction pathways that may be kinetically controlled by varying either the concentration of reducing agent or the extent of L4 oxidation. Comparison of selected ion chronograms indicates that Au2(L4)2H+ may be an intermediate in the formation of Au8(L4)42+ and Au10(L4)52+ while a variety of chlorinated clusters may be involved in the formation of Au9(L4)4H2+. Additionally, high resolution mass spectrometry enabled the identification of 53 new gold containing species produced under highly oxidative conditions. New intermediate species were identified which aid the understanding of how different size gold clusters may be stabilized during the growth process.


 Please wait while we load your content...
Please wait while we load your content...