An FTIR emission study of the products of NO A2Σ+ (v = 0, 1) + O2 collisions
Abstract
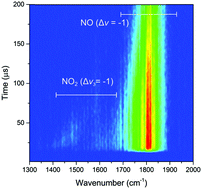
Collisional quenching of NO A2Σ+ (v = 0, 1) by O2 has been studied through the detection of vibrationally excited products by time-resolved Fourier transform infrared emission spectroscopy. Non-reactive quenching of NO A2Σ+ (v = 0) produces a vibrational distribution in NO X2Π which has been quantified for v = 2–22, and is found to be bimodal. The results are consistent with two quenching channels. The first forms the ground X3Σ−g or low-lying a 1Δg electronic state of O2 with a distribution including high vibrational levels of NO X2Π which is slightly hotter than statistical. Two possibilities are identified for the second channel. The first, with a similar quantum yield to that producing higher vibrational levels, forms a highly electronically excited state, such as O2 c1Σ−u, with low vibrational levels in NO X2Π which are inverted with a distribution resembling that resulting from a sudden or harpoon mechanism. The second is that ground state oxygen is formed with low vibrational energy partitioned into NO X2Π. In addition, vibrationally excited NO2 is observed, but at intensities which indicate that it is formed in low quantum yield. Quantitatively unobservable processes (defined as those which do not form ground state NO (v ≥ 2)) are found to have a branching ratio of at most 25 ± 5%. The results are compared with those of previous studies and the most consistent interpretation suggests that dissociation of O2 to form ground state O(3P) atoms and ground vibrational state NO X2Π (v = 0) is the main reactive process rather than NO2 formation. Qualitatively similar results are seen for the quenching of NO A2Σ+ (v = 1).


 Please wait while we load your content...
Please wait while we load your content...