Probing the bonding of CO to heteronuclear group 4 metal–nickel clusters by photoelectron spectroscopy†
Abstract
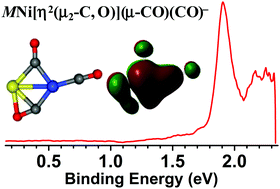
A series of heterobinuclear group 4 metal–nickel carbonyls MNi(CO)n− (M = Ti, Zr, Hf; n = 3–7) has been generated via a laser vaporization supersonic cluster source and characterized by mass-selected photoelectron velocity-map imaging spectroscopy. Quantum chemical calculations have been carried out to elucidate the geometric and electronic structures and support the spectral assignments. The n = 3 cluster is determined to be capable of simultaneously accommodating three different types of CO bonds (i.e., side-on-bonded, bridging, and terminal modes), resulting in a MNi[η2(μ2-C, O)](μ-CO)(CO)− structure, which represents the smallest metal carbonyl with the involvement of all the main modes of metal–CO coordination to date. The building block of three bridging CO molecules is favored at n = 4, the structure of which persists up to n = 7. The additional CO ligands are bonded terminally to the metal atoms. The present findings provide important new insight into the structure and bonding mechanisms of CO molecules with heteronuclear transition metals, which would have important implications for understanding chemisorbed CO molecules on alloy surfaces.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...