Co2SnO4 nanoparticles as a high performance catalyst for oxidative degradation of rhodamine B dye and pentachlorophenol by activation of peroxymonosulfate†
Abstract
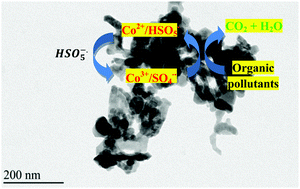
Spinel Co2SnO4 nanoparticles are synthesized by a facile hydrothermal route in alkaline solution using SnCl4 and CoCl2 as precursors. The structure, morphology and chemical composition of the nanoparticles are characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), energy dispersive X-ray (EDX), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS) and thermogravimetric analysis (TGA). The catalytic performance of the Co2SnO4 nanoparticles is thoroughly evaluated for peroxymonosulfate (PMS) activation for removal of rhodamine B (RhB) and pentachlorophenol (PCP) from water. The influence of different process parameters on the RhB degradation efficiency is examined and the catalytic stability is evaluated. Under optimized conditions, the Co2SnO4/PMS system is very efficient with a full degradation of RhB and PCP in less than 10 min at room temperature, as revealed by high performance liquid chromatography (HPLC) analysis. Quenching experiments suggested that sulfate radicals (SO4˙−) are the main active species in the degradation process. Moreover, the Co2SnO4 catalyst is stable without any apparent activity loss after 5 cycling runs.


 Please wait while we load your content...
Please wait while we load your content...