Free-energy patterns in inclusion complexes: the relevance of non-included moieties in the stability constants
Abstract
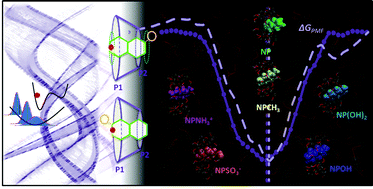
Inclusion complexes play a definite role in a variety of applications, ranging from drug solubilization to smart materials. This work presents a series of studies based on molecular dynamics, including potential of mean force calculations, and aiming at understanding the factors that govern inclusion. Naphthalene and its derivatives are used as guests for a common host, β-cyclodextrin. It is observed that the substitution of naphthalene promotes an increase in the complexation constant (up to 100-fold), irrespective of the nature of the substituent, the latter comprising small hydrophobic and hydrophilic (including charged) groups. It is also seen that entropy does not favor inclusion, the order of magnitude of the binding free energy being given by the enthalpic component, with a dominating guest–host interaction contribution. Desolvation penalizes the inclusion process, and is not observed in the vicinity of the hydrophilic and charged groups, which remain exposed to the solvent. Results suggest that substantial modulation of the inclusion complexes can be achieved imposing different substituents, with direct transposition for the modulation of properties in supramolecular structures based on these complexes.


 Please wait while we load your content...
Please wait while we load your content...