Gold-supported two-dimensional cobalt oxyhydroxide (CoOOH) and multilayer cobalt oxide islands
Abstract
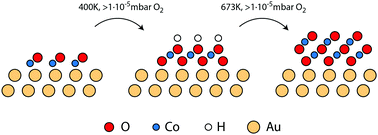
In the present study, we investigate the facile conversion of Co–O bilayer islands on a Au(111) surface into preferentially O–Co–O trilayers in an oxygen atmosphere and O–Co–O–Co–O multilayers at elevated temperature. We characterize and compare the island morphologies with scanning tunneling microscopy, X-ray photoemission spectroscopy (XPS) and valence band spectroscopy, and show that the cobalt oxidation state changes from Co2+ in bilayers to purely Co3+ in trilayers and a mixture of Co2+ and Co3+ in the multilayer morphology. In contrast to bilayers and multilayers, the trilayer structure appears to grow pseudomorphic with the Au(111) substrate, and in addition we reveal the presence of a hydroxyl overlayer on this island type as evidenced by the appearance of a 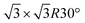 superstructure in STM correlated with the fingerprints of OH species in XPS and valence band spectroscopy. The obtained layered morphology consisting of hydroxylated trilayer islands is identical to an exfoliated sheet of the β-CoOOH which is proposed to be the active phase of the cobalt oxide oxygen evolution reaction catalyst present in the electrochemical environment, and we note that this synthesized structure thus could serve as a valuable model catalyst.
superstructure in STM correlated with the fingerprints of OH species in XPS and valence band spectroscopy. The obtained layered morphology consisting of hydroxylated trilayer islands is identical to an exfoliated sheet of the β-CoOOH which is proposed to be the active phase of the cobalt oxide oxygen evolution reaction catalyst present in the electrochemical environment, and we note that this synthesized structure thus could serve as a valuable model catalyst.


 Please wait while we load your content...
Please wait while we load your content...