Comparison of tetrel bonds in neutral and protonated complexes of pyridineTF3 and furanTF3 (T = C, Si, and Ge) with NH3†
Abstract
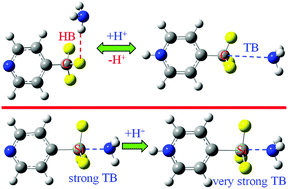
Ab initio calculations have been performed for the complexes H+–PyTX3⋯NH3 and H+–furanTF3⋯NH3 (T = C, Si, and Ge; X = F and Cl) with focus on geometries, energies, orbital interactions, and electron densities to study the influence of protonation on the strength of tetrel bonding. The primary interaction mode between α/β-furanCF3/p-PyCF3 and NH3 changes from an F⋯H hydrogen bond to a C⋯N tetrel bond as a result of protonation. Importantly, the protonation has a prominent enhancing effect on the strength of tetrel bonding with an increase in binding energy from 14 to 30 kcal mol−1. The tetrel bonding becomes stronger in the order H+–p-PySiF3⋯NH3 < H+–m-PySiF3⋯NH3 < H+–o-PySiF3⋯NH3, showing a reverse trend from that of the neutral analogues. In addition, there is competition between the tetrel and hydrogen bonds in the protonated complexes, in which the hydrogen bond is favored in the complexes of H+–p-PyCF3 but the tetrel bond is preferred in the complexes of H+–p-PyTX3 (T = Si, Ge; X = F, Cl) and H+–o/m-PySiF3.


 Please wait while we load your content...
Please wait while we load your content...