Kinetic analysis of non-isothermal solid-state reactions: multi-stage modeling without assumptions in the reaction mechanism
Abstract
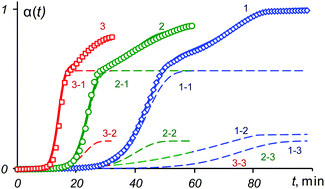
A novel non-linear regression method for modeling non-isothermal thermogravimetric data is proposed. Experiments for several heating rates are analyzed simultaneously. The method is applicable to complex multi-stage processes when the number of stages is unknown. Prior knowledge of the type of kinetics is not required. The main idea is a consequent estimation of parameters when the overall model is successively changed from one level of modeling to another. At the first level, the Avrami–Erofeev functions are used. At the second level, the Sestak–Berggren functions are employed with the goal to broaden the overall model. The method is tested using both simulated and real-world data. A comparison of the proposed method with a recently published ‘model-free’ deconvolution method is presented.


 Please wait while we load your content...
Please wait while we load your content...