Picosecond activation of the DEACM photocage unravelled by VIS-pump-IR-probe spectroscopy†
Abstract
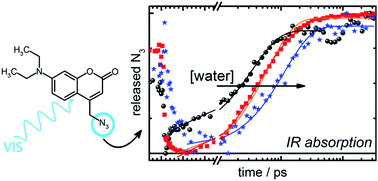
The light-induced ultrafast uncaging process of the [7-(diethylamino)coumarin-4-yl]methyl (DEACM) cage is measured by time-resolved visible-pump-infrared-probe spectroscopy, and supported by steady-state absorption spectroscopy in the visible and infrared spectral regions. Understanding the uncaging process is important because its favorable properties make DEACM an interesting case for chemical and biological applications. It has a convenient absorption in the visible spectral range, and is relatively easily modified to carry leaving groups (LGs) such as nucleotides, substrates or inhibitors, which are inactive when bound and active when released. Previous work suggested a lower limit for the uncaging rate, which places it among the fastest available cages. Here, we determine the photodissociation directly to occur on the picosecond time scale by monitoring the appearance of the released LG in the infrared spectral region. In the present study, azide (N3) is chosen as an LG to monitor photodissociation because its vibrational mode is spectrally isolated (hence easy to follow) and its absorption wavenumber is sensitive to local structural rearrangements. The uncaging process is recorded up to 3 nanoseconds and compared to the collected steady-state spectra. The free LG appears on a picosecond time scale, rendering this one of the fastest known cages. No evidence is found for a tight-ion pair (TIP) preceding the free LG. The uncaging mechanism is found to be slowed down upon the addition of water to acetonitrile.


 Please wait while we load your content...
Please wait while we load your content...